Cool Info About Periodic Table Cgp Homophones Flashcards Pdf

Below are some useful videos and a knowledge organiser for revising the c1 chemistry topic on atomic structure and the periodic table.
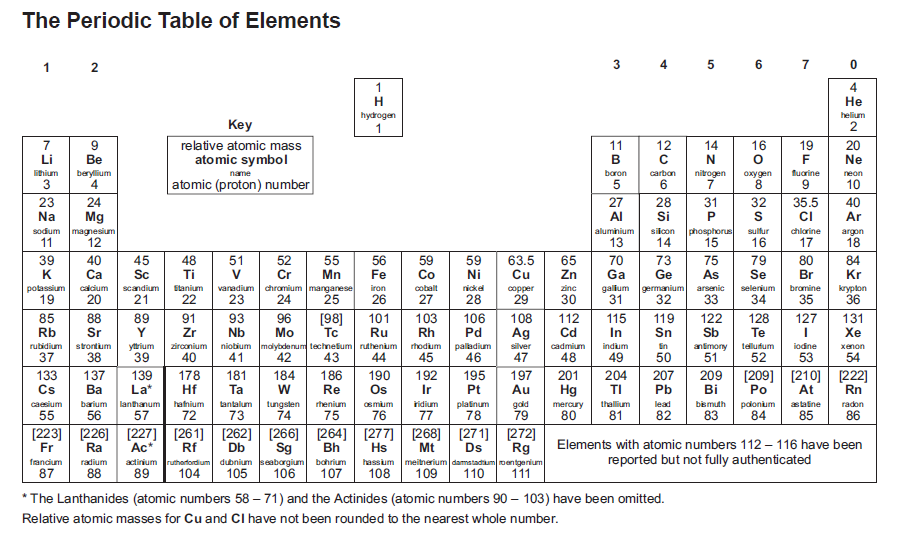
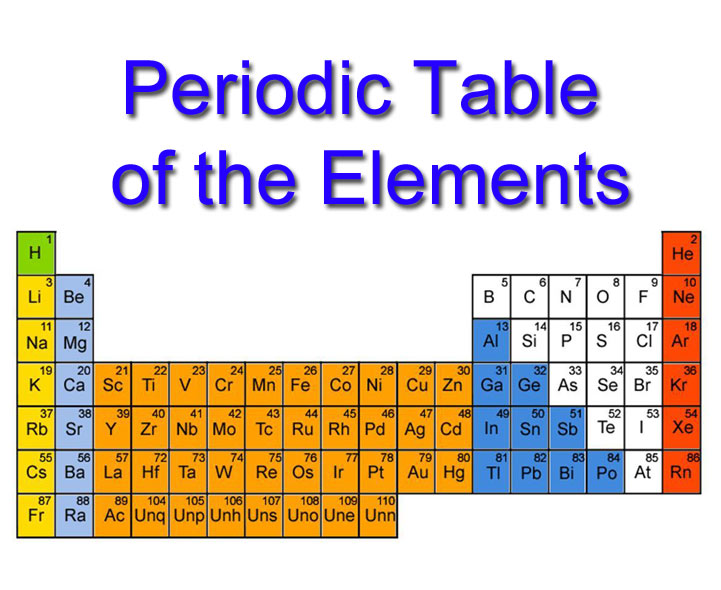
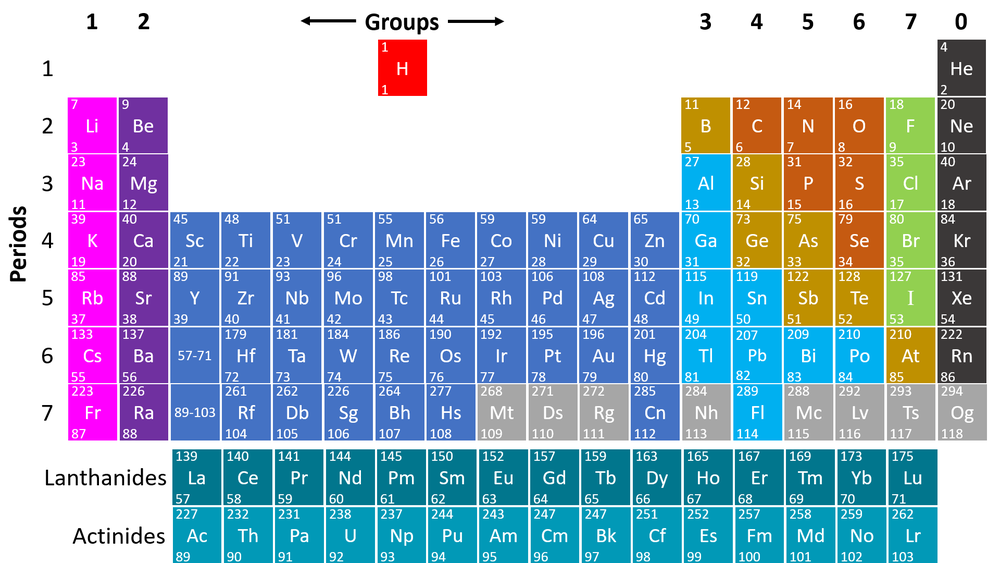
Periodic table cgp. When the chemical elements are thus arranged, there is a recurring pattern called the “periodic law” in their properties, in which elements in the same column (group. Look up chemical element names, symbols, atomic masses and other properties, visualize trends, or even test your elements knowledge by playing a periodic table game! Periodic table, in chemistry, the organized array of all the chemical elements in order of increasing atomic number —i.e., the total number of protons in the atomic nucleus.
The arrangement of elements is based on their structure and properties. Study with quizlet and memorize flashcards containing terms like what does the group number tell you?, what are the group 1 elements called?, what are the group 7 elements called? The numbers of subatomic particles in an atom can be calculated from its atomic.
It has a unique arrangement of rows and columns. Elements are listed on the periodic table. The group number increases by one for every additional column, up to a maximum of 18.
Electronic structures model how electrons are arranged in atoms. In the modern periodic table, elements are in order of atomic number in. Periods 6 and 7 have 32 elements,.
The periodic table is a way of organising the elements which is used by scientists to group elements with similar properties. Where do we find the mass number in the periodic table? Interactive periodic table suitable for a level chemistry students.
Periods 4 and 5 have 18 elements. It is an icon of chemistry and is widely used in physics and other sciences. Interactive periodic table showing names, electrons, and oxidation states.
The table is called a periodic Above the element abbreviation and to the left (the larger number) how do we find the number of neutrons from mass and atomic number? The periodic table, also known as the periodic table of the elements, arranges the chemical elements into rows (periods) and columns (groups).
Click the card to flip 👆 in order of atomic weight click the card to flip 👆 1 / 10 flashcards learn test match created by teeeejeeeee students also viewed pote 35 terms mary_lepauloue27 preview memorization quiz 26 terms mary_wick7 Are arranged in a chart called the periodic table. In addition to these, the cgp and collins revision guides contain everything you need to know for your upcoming exam:
4.1.2 the periodic table 4.1.2.1 the periodic table the elements in the periodic table are arranged in order of atomic (proton) number and so that elements with similar properties are in columns, known as groups. Represent the electronic structures of the first twenty elements of the periodic table in both forms. Including revision guides, revision cards, workbooks and more!
In the modern periodic table, elements are in order of atomic number in periods and groups. It includes electronic configurations, melting points and boiling points. Period 1 has only two elements (hydrogen and helium), while periods 2 and 3 have 8 elements.